Collyer’s seventh claim peddles the old canard that there are no ‘half formed eyes’ and that organs appear fully formed: “All known species (several million) appear fully developed with all vital organs fully operational. There are no part-formed eyes, half-developed intestines or evolving feathers.” Once again, Collyer is completely wrong.
The natural world is replete with examples of half-formed organisms and biochemical systems. The nautilus, a relative of the octopus has a ‘half-formed eye’ to use Collyer’s phrase in that its eye is missing a lens. In fact, some species of blind moles still develop vestigial eyes that do not work. Many beetle species have fused outer wings that cover useless inner wings. Humans and apes have a broken vitamin c biosynthetic pathway that is missing an enzyme that would allow them to make their own vitamin C, like other animals do.
The fossil record shows the evolution of feathers from their origin in non-avian dinosaurs. Once again, Collyer's claims fall apart when critically examined.
Collyer has not made it clear as
to whether he is referring to extinct species or extant species. If we restrict ourselves to currently living species, one
can readily provide examples of suboptimal design, ranging from gross anatomy
down to molecular biology. If we look at the fossil record, then we can see the evolution of structures such as feathers. In either
case, he is wrong.
Furthermore, he has ignored the obvious point that if an organism does not have functioning ‘vital organs’ then it won’t be alive. Rather, the question should be whether systems and organs are optimally configured. Evolution doesn’t care whether something is perfect, but rather whether it confers a differential survival advantage. Therefore, one would fully expect to see sub-optimal design, as well as evidence of contingency secondary to evolutionary history.
Examples of suboptimal design abound. Furthermore, we also see evidence of shared suboptimal design in nested hierarchies (groups within groups) which is what one would expect if the original design plan was laid down early in evolutionary history, and inherited by the descendants of the original species.
Furthermore, he has ignored the obvious point that if an organism does not have functioning ‘vital organs’ then it won’t be alive. Rather, the question should be whether systems and organs are optimally configured. Evolution doesn’t care whether something is perfect, but rather whether it confers a differential survival advantage. Therefore, one would fully expect to see sub-optimal design, as well as evidence of contingency secondary to evolutionary history.
Examples of suboptimal design abound. Furthermore, we also see evidence of shared suboptimal design in nested hierarchies (groups within groups) which is what one would expect if the original design plan was laid down early in evolutionary history, and inherited by the descendants of the original species.
The vertebrate eye is the classic example, with problems abounding as a direct
result of its design. Light cells in the retina point backwards, away
from the light. From a design perspective, this is clearly a suboptimal
solution as one would normally point the light sensing cells towards the light.
Any creationist argument that claims this is somehow a good solution is negated
by the simple fact that in nature, a far superior solution exists – the
cephalopod eye. Here, the light sensing cells point towards the light, and are
at the top of the retina. One is entitled to wonder why sperm whales do not
have the same intelligent retinal configuration that the giant squid on which
they prey have.
One problem directly arising from
this design is the existence of a blind spot, which arises from the need
for nerves and blood vessels to exit and enter the eye. In order to make room,
the section of the retina where this occurs has no light sensitive cells.
Our brains edit out this blind
spot so we are not aware of it unless specifically looking for it, but it is a
direct reminder of how suboptimal – and unnecessary – this is. The cephalopods
do not have a blind spot, as the light sensing cells are in front of the nerve
fibres and blood vessels.
Other problems arising from
having blood vessels in front of the light sensing cells, as the neurologist
Steve Novella points out [1] include the vulnerability of vision to disease
such as diabetic retinopathy, which occurs when blood vessels in the retina
proliferate in response to chronically low oxygen levels. These blood vessels
obscure vision, and need to be burned away with lasers. Even a small retinal
haemorrhage will markedly affect vision, as will any inflammatory changes in
the cells before the light sensing part of the retina.
To these problems one can also add retinal detachment which occurs when the light-sensing layer of the retina detaches from the back of the eye. Cephalopod eyes do not suffer this problem as the light sensing cells are firmly tethered to the underlying layer. Macular degeneration, which is the most common cause of blindness, is also a direct function of this suboptimal design. The macula is a section of the retina which by virtue of its extremely high density of rods and cones – achieved by moving away nerves and blood vessels to allow this increased density of receptor cells – allows more detailed vision to occur. Degenerative change in this part of the retina destroys the area with the greatest visual acuity and causes significant functional impairment. If we had a retina with photo-sensing cells in front of the nerves and blood vessels, there would be no need for a macula to compensate for the vertebrate retinal design.
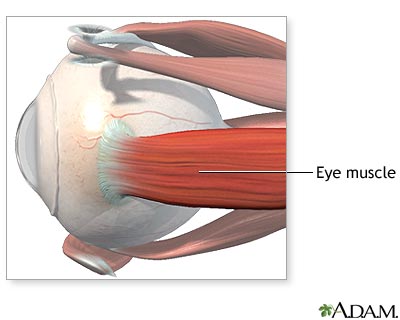
The extraocular muscles that move the eye, and the cranial nerves that innervate them are also evidence for evolution. Novella is worth quoting in detail to show why:
The arrangement of the extraocular muscles—the muscles that move the eyes—is also difficult to explain without appealing to evolutionary contingency. There are more muscles than are minimally necessary and yet there is no functional redundancy. In order to move a sphere in any direction, only three muscles would be necessary, evenly spaced like the legs of a tripod. The human eye has six—the superior, inferior, lateral, and medial rectus, and the superior and inferior oblique. And yet, despite the extra three muscles, the loss of function of any one muscle causes an impairment of eye movement and results in double vision or displaced vision. A more frugal design with only three muscles would be more efficient and less prone to malfunction, as there are fewer components to break down. If the eye were to be designed with more than the minimal three muscles, then it would make sense to arrange the muscles so that the loss of one or even more would not impair eye movement.
Fossil evidence suggests that primitive jawed fishes had seven extraocular muscles. Some modern vertebrates retain this configuration, such as dogs that have a 7th extraocular muscle, the retractor bulbi, not found in humans, although there is a report of an incidence of the retractor bulbi occurring in a human, a likely recitavistic trait.
The configuration of cranial nerve control of the extraocular muscles also makes no design sense. The lateral rectus is controlled by cranial nerve VI (abducens), the superior oblique by cranial nerve IV (trochlear), and the rest by cranial nerve III (oculomotor). There is no functional advantage to this particular arrangement; it is an accident of evolution. Having three cranial nerves responsible for eye muscles multiplies the opportunity for failure of any one, and again there is no redundancy as a hedge against malfunction.
But the most suboptimal aspect of cranial nerve innervation of the extraocular muscles is the abducens, cranial nerve VI. The abducens takes an unnecessarily long path from the brainstem, through the skull, and to the lateral rectus. There is no design reason for this long path—it too is an accident of evolution. It makes the abducens particularly vulnerable to injury or stretching, and for this reason, abducens palsy is the most common extraocular muscle weakness. The trochlear nerve (cranial nerve IV to the superior oblique) also takes an unusual pathway—it exits the brainstem heading toward the back of the brain, which is the wrong direction. It must then swing around and head toward the eyes. As with the abducens nerve, this unnecessarily long pathway increases the potential for malfunction. [2]
Examining the pathway taken by
these cranial nerves in human cadaver brains reinforced this fact to me when I
studied neuroanatomy – it simply made no sense other than as a function of
evolutionary history.
Things are not all perfect among the cephalopods however. The eye of the nautilus is hardly optimal. While it has a sensitive retina, it has no lens, relying instead on a pinhole mechanism to focus light. Other cephalopods such as cuttlefish, octopuses and squid have a lens. By any objective measure, the nautilus eye is incomplete:
Things are not all perfect among the cephalopods however. The eye of the nautilus is hardly optimal. While it has a sensitive retina, it has no lens, relying instead on a pinhole mechanism to focus light. Other cephalopods such as cuttlefish, octopuses and squid have a lens. By any objective measure, the nautilus eye is incomplete:
The retina consists of some 4x10^6 close-set rhabdomeric photoreceptors. As these photoreceptors are only some 5-10 μm in diameter the retina should be capable of good vision. However, it has been calculated that the pinhole only provides a 2.3 [degree] minimal resolvable angle which translates...to a lateral distance across the retina of no less than about 0.4mm. Nautilus seems to have developed a retina far better than its optics can use! Indeed, investigation of the animal’s lifestyle shows that it spends much of its time in light of low intensity, descending to a depth of some 300m during the day and ascending to about 150m at night. Why have such an excellent retina if it is never used? The Nautilus eye remains a mystery. [3]
One is justifiably entitled to ask why the nautilus does not have a lens when the other cephalopods have one. If Collyer wants an example of an
incomplete eye, then the nautilus eye, with its pinhole camera design certainly
fits the bill.
In fact, one does not have to
look far to find examples in nature, which can be found
from gross anatomy down to molecular biology. One textbook example is the
pathway taken by the recurrent laryngeal nerve in mammals. This is a branch of
the vagus nerve, one of the 12 cranial nerves - so called because instead of
branching from the spinal cord, they branch from the brain stem, and exit via
other foramina. The recurrent laryngeal nerve is a branch of the vagus nerve
and is one of the nerves that innervate the larynx. From its origin off the
vagus nerve to its destination at the larynx is a distance of only a few
centimetres. However, instead of passing directly to the larynx, it takes a
totally pointless detour down the neck into the chest, loops under the aorta
(the largest artery in the body, which emerges directly from the left ventricle
of the heart) and then ascends up the neck to the larynx. There is no reason
for this detour.
This by itself does not prove common descent or evolution. However, if the same design is found in multiple organisms in the same group, then the idea of common descent becomes a far more parsimonious explanation. One would expect a feature in an ancestral species to be inherited by its descendants. Special creation is forced to posit multiple independent creation events with the same feature being repeated every time. Again, God can create an animal with a nerve directly passing to the larynx - there is absolutely no physiological need for this detour. Occam's razor would suggest common descent as the most parsimonious explanation.
The giraffe is the most spectacular demonstration of the path of the recurrent
laryngeal nerve. As with other mammals, it goes past the larynx, down its long
neck, under its aorta and up to the larynx. Mark Ridley explains the reason
behind this:
Evolution by natural selection proceeds in small, local steps and each change has to be advantageous in the short term. Unlike a human designer, natural selection cannot favour disadvantageous changes in the knowledge that they will ultimately work out for the best. As Wright emphasised in his shifting balance model...natural selection may climb to a local optimum, where the population may be trapped because no small change is advantageous, though a large change could be. As we saw, selection itself (when considered in a fully multidimensional context), or neutral drift, may lead the population away from local peaks; but it also may no. Some natural populations now may be imperfectly adapted because the accidents of history pointed their ancestors in what would later become the wrong direction.
The recurrent laryngeal nerve provides an amazing example. The laryngeal nerve is, anatomically, the fourth vagus nerve, one of the cranial nerves. These nerves first evolved in fish-like ancestors. As figure 10.12a shows, successive branches of the vagus nerve pass, in fish, behind the successive arterial arches that run through the gills. Each nerve takes a direct route from the brain to the gills. During evolution, the gill arches have been transformed; the sixth gill arch has evolved in mammals into the ductus arteriosus, which is anatomically near to the heart. The recurrent laryngeal nerve still follows the route behind the (now highly modified) "gill arch": in a modern mammal, therefore, the nerve passes from the brain, down the neck, round the dorsal aorta, and back up the larynx.
In humans, the detour looks absurd, but it is only a distance of a foot or two. In modern giraffes, the nerve makes the same detour, but it passes all the way down and up the full neck of the giraffe's neck. The detour is almost certainly unnecessary and probably imposes a cost on the giraffe (because it has to grow more nerve than necessary and signals sent down the nerve will take more time and energy). Ancestrally, the direct route for the nerve was to pass posterior to the aorta; but as the neck lengthened in the giraffe's evolutionary lineage the nerve was led on a detour of increasing absurdity. If a mutant arose in which the nerve went directly from brain to larynx, it would probably be favoured (though the mutation would be unlikely if it required a major embryological reorganization); the imperfection persists because such a mutation has not arisen (or it arose and was lost by chance). The fault arose because natural selection operates in the short term, with each step taking place as a modification of what is already present. This process can easily lead to imperfections due to historical constraint - though most will not be as dramatic as the giraffe's recurrent laryngeal nerve. [4]
Evolution is constrained - it
cannot work from a fresh sheet, but starts with what it is given, which in this
case is the basic fish-like anatomy, which explains neatly the origin of this
quirk. With special creation, there is no constraint, so design can be as
elegant and efficient as possible. The fact that all mammals have this feature is difficult at best to square with special creation, but
follows easily from common descent.
Other anatomical examples
include:
Dewclaws. These are vestigial
digits that can be found on the feet of many vertebrates. In animals that walk
on their digits such as dogs and cats, they often do not reach the ground.
Wing of the kiwi: this bird is flightless, yet it has a vestigial wing
which isn't even of use as a stabilising device when running. From an
evolutionary point of view, this half-formed useless wing makes sense as
the kiwi it the descendant of birds that could fly, but which over time
lost the ability to fly.
Vestigial eyes in moles: despite the fact that these animals spend most of their time underground and therefore reply on other sensory modalities such as smell and touch, many of these species have vestigial eyes. Again, if these animals were specially created, the addition of useless eyes is inexplicable. Conversely, their presence is perfectly understandable in the light of evolution given that the ancestors of moles once had functioning eyes.
Piloerection. This reflex occurs either during cold weather or in response to stress. In the latter, it causes animal to appear larger and more intimidating, while in the former it traps an insulating layer of air. In humans, the response serves neither function and is a relic of our animal ancestry.
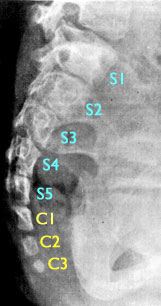
Coccyx. Otherwise known as the
tail bone. During embryogenesis, human embryos grow a tail, which later
degenerates into the coccyx. Its only function now is to serve as an attachment
for muscles. The genes for making a tail still exist in humans, and examples of
functional tails that contain muscle, nerves and are capable of movement are documented in the literature.
A radiogram of the sacral region of a six-year old girl with an atavistic tail. The tail was perfectly midline and protruded form the lower back as a soft appendage. The five normal sacral vertebrae are indicated in light blue and numbered; the three coccygeal tail vertebrae are indicated in light yellow. The entire coccyx (usually three or four tiny fused vertebrae) is normally the same size as the fifth sacral vertebrae.
Tooth development in baleen
whales. Despite not having teeth, baleen whale embryos grow tooth buds in utero,
only to break them down again later in embryogenesis. Transitional fossils of
whales with both teeth and baleen-related structures exist, so we know baleen
whales evolved from toothed whales. Molecular evidence corroborates this fact
as modern baleen whales have broken genes that code for enamel-related protein.
[5] The presence of broken genes coding for enamel-related protein in whales
that do not have teeth, not to mention the embryonic development of tooth buds
that are later broken down is very much an example at the gross anatomical and
molecular level of a half-built structure. From an evolutionary point of view,
it is readily understandable, but from the perspective of special creation, it is
inexplicable.
Dissected fin whale (a baleen whale) showing tooth buds which develop only to later break down. Source: Deméré T.A. et al “Morphological and Molecular Evidence for a Stepwise Evolutionary Transition from Teeth to Baleen in Mysticete Whales” Syst Biol (2008) 57 (1): 15-37
Palmaris longus. in humans, this
upper limb tendon which lies between the flexor carpi ulnaris and the flexor
carpi radialis muscles has no appreciable function, and in fact is absent in
between 10-15% of people. [6] It is often harvested as a tendon graft in hand
surgery without any functional deficit. It functions in other animals to expose
claws – in humans, this function is no longer necessary and this muscle has
become vestigial.
Plantaris. this lower limb muscle
has minimal functionality and is absent in up to 10% of the population. As with
the palmaris longus, it is often harvested for use in reconstruction surgery
with no marked functional deficit, as plantarflexion of the foot is carried out
mainly by the gastrocnemius and soleus muscles. In primates, it is used for
grasping with the feet, but as we are an obligate bipedal species, we have lost
the ability to grasp with the feet and no longer need this function. Again,
this is very much an example of a vestigial, “half-functional” structure whose
existence makes sense in the light of evolution.
Apomixis in flowering plants.
this refers to asexual reproduction via seeds, and is found in a number of
plant genera including the dandelions. Put simply, these plants have flowers,
despite the fact they do not need them for reproduction. Flowers in apomictic
plants are very much a vestigial structure.
Toenails in manatees. these
aquatic mammals have flippers and no separate digits, but still retain
toenails.
Flightless beetles. there are
many flightless beetle species such as the Kauai Flightless Stag Beetle [7]
which have fused outer wing covers, but still have inner wings present in a
vestigial state. If these beetles were designed from scratch, the presence of
useless membranous wings under their fused outer wing covers would be regarded
as pointless. Recognition of their evolution from beetles that
could fly however makes this understandable.
Brachypterous Colophon
westwoodi with vestigial wing visible. [image M. Cochrane, Iziko © ]
One could go on, but the falsity of Collyer’s statement has been adequately demonstrated, as well as the paucity of research behind it. One final comment should be made about his assertion that no “evolving feathers” exist. Fossil evidence [8-11] of feathered dinosaurs exists, providing more evidence to the mainstream palaeontological view that birds evolved from dinosaurs and in fact are classed as dinosaurs. Feathers were not created specifically for avian flight, insulation and decoration, but evolved in dinosaurs. Although the fossilisation process has created artefacts [12] which makes detecting an evolutionary trend in feather evolution difficult, this does not detract substantially from previously documented models of feather evolution. [13]
Three-dimensional virtual reconstruction of a fossil feather from the Early Cretaceous (about 100 million years ago) preserved in amber. This feather could be from either a bird or a non-avian theropod. (a-c) long barbs form two vanes on each side of a relatively flattened shaft; (d) the shaft is flattened and composed of incompletely fused bases of the barbs, a stage in feather evolution that was hitherto unknown in fossil records and corresponding to an intermediate stage between the very distinct stages II and IIIa defined by Prum (1999). Scale bars, 100 µm Proc. R. Soc. B 22 May 2008 vol. 275 no. 1639 1197-1202
Feathers from French amber take place between the very distinct stages II and IIIa proposed in the developmental model of Prum. Proc. R. Soc. B 22 May 2008 vol. 275 no. 1639 1197-1202
In fact, recent fossil feather discoveries have shown that current theories on
feather evolution are definitely on the right track:
According to developmental theories, the rachis is the result of a complete fusion of the barbs, even in down feathers with a small basic rachis, and a planar form of feathers results from the helical growth of barb ridges within the follicle and interlocking between neighbouring barbs to create the vane. A shaft consisting of incompletely fused, still distinguishable, partially superimposed barbs is not considered in feather evolution, although this stage would logically have existed with regard to the formation of feathers in the follicle. The structure observed in these specimens from French amber therefore represents the first fossil evidence of the intermediate stage between the very distinct stages II and IIIa defined by Prum in his theory of evolutionary diversification of feathers. Stage II is characterized by non-ramified barbs attached at their base to the calamus, without barbules. Stage IIIa corresponds to the appearance of a central shaft formed by the fusion of non-ramified barbs and the appearance of the planar form. Stage IIIb exhibits barbules without differentiation between basal or distal part of the feather, unlike in stage IV. Similarly, the new fossils take place between stages II and III defined by Xu, and more precisely correspond to the early phase of stage III. The present discovery, therefore, sheds new light on the idealized nature of the developmental stages of feather evolution. [14]
Feather evolutionary-developmental model, terminology, and stage I and II specimens from Canadian amber. (A) Current evolutionary-developmental model for feathers consists of a stage I morphology characterized by a single filament: This unfurls into a tuft of filaments (barbs) in stage II. In stage III, either some tufted barbs coalesce to form a rachis (central shaft) (IIIa), or barbules (segmented secondary branches) stem from the barbs (IIIb); then, these features combine to produce tertiary branching (IIIa+b). Barbules later differentiate along the length of each barb, producing distal barbules with hooklets at each node to interlock adjacent barbs and form a closed pennaceous (vaned) feather (stage IV). Stage V encompasses a wide range of additional vane and subcomponent specializations. Most modern birds possess stage IV or V feathers or secondary reductions from these stages Green, calamus or equivalent; blue, barbs; purple, rachis; red, barbule internodes; d.b., distal barbules; r., ramus; p.b., proximal barbules. (B) Field of filaments cut obliquely (stage I). (C) Filament clusters variably oriented (stage II). (D) Close-up of (C), showing filaments that comprise clusters. Pigmentation coupled with comparatively thick outer walls produces darker color than in isolated filaments. Scale bars, (B) and (C) 1 mm, and (D) 0.1 mm (Text from http://people.eku.ed..._evolution.htm. Images from McKellar R et al "A Diverse Assemblage of Late Cretaceous Dinosaur and Bird Feathers from Canadian Amber" Science (2011) 333(6049):1619-1622
Contrary to Collyer’s assertion, we do have evidence of evolving feathers which corroborate theories of feather evolution.
Conclusion
Collyer has once again made a sweeping claim which when examined turns out to be completely without basis. Half-formed organs abound in nature such as the eye of the nautilus, the vestigial wing of the kiwi and the half-formed inner wings of flightless beetles. All these examples make sense in the light of evolution.
Perhaps the most damning indictment of Collyer's 'fact' is his claim about the evolution of feathers, where fossil discoveries have shown that contrary to special creationist claims, feathers have indeed evolved.
References
1. Novella S “Suboptimal Optics: Vision Problems as Scars of Evolutionary History” Evo Edu Outreach (2008) 1:493-497
2. ibid, p 496.
3. Smith. C.U.M. “Biology of Sensory Systems” (2008, Wiley-Blackwell) p 267
3. Smith. C.U.M. “Biology of Sensory Systems” (2008, Wiley-Blackwell) p 267
4. Ridley M "Evolution" (2004, Wiley-Blackwell) p 281-2
5. Deméré T.A. et al “Morphological and Molecular Evidence for a Stepwise Evolutionary Transition from Teeth to Baleen in Mysticete Whales” Syst Biol (2008) 57 (1): 15-37
6. Sebastin SJ, Puhaindran ME, Lim AY, Lim IJ, Bee WH (October 2005). "The prevalence of absence of the palmaris longus--a study in a Chinese population and a review of the literature". Journal of Hand Surgery 30 (5): 525–527
8. Xu, X., Q. Zhao, M. Norell, C. Sullivan, D. Hone, G. Erickson, X. L. Wang et al. (2009). "A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin". Chinese Science Bulletin 54: 430–435.
9. Hu, D. Y., L. H. Hou, L. J. Zhang, and X. Xu (2009). "A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus". Nature 461 (7264): 640–643
10. Xu, Xing & Fucheng Zhang (2005). "A new maniraptoran dinosaur from China with long feathers on the metatarsus". Naturwissenschaften 92 (4): 173–177
11. Xu, Xing (2006). "Feathered dinosaurs from China and the evolution of major avian characters". Integrative Zoology 1 (1): 4–11.
12. Foth, C. (2011). "On the identification of feather structures in stem-line representatives of birds: evidence from fossils and actuopalaeontology." Paläontologische Zeitschrift, (advance publication) doi:10.1007/s12542-011-0111-3
13. Xu, X.; Guo, Y. (2009). "The origin and early evolution of feathers: insights from recent paleontological and neontological data". Vertebrata PalAsiatica 47 (4): 311–329.
14. Perrichot V, Marion L, Néraudeau D, Vullo R, Tafforeau P. The early evolution of feathers: fossil evidence from Cretaceous amber of France. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1639):1197.
9. Hu, D. Y., L. H. Hou, L. J. Zhang, and X. Xu (2009). "A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus". Nature 461 (7264): 640–643
10. Xu, Xing & Fucheng Zhang (2005). "A new maniraptoran dinosaur from China with long feathers on the metatarsus". Naturwissenschaften 92 (4): 173–177
11. Xu, Xing (2006). "Feathered dinosaurs from China and the evolution of major avian characters". Integrative Zoology 1 (1): 4–11.
12. Foth, C. (2011). "On the identification of feather structures in stem-line representatives of birds: evidence from fossils and actuopalaeontology." Paläontologische Zeitschrift, (advance publication) doi:10.1007/s12542-011-0111-3
13. Xu, X.; Guo, Y. (2009). "The origin and early evolution of feathers: insights from recent paleontological and neontological data". Vertebrata PalAsiatica 47 (4): 311–329.
14. Perrichot V, Marion L, Néraudeau D, Vullo R, Tafforeau P. The early evolution of feathers: fossil evidence from Cretaceous amber of France. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1639):1197.



.jpg/800px-Nautilus_pompilius_(head).jpg)