Heterochronic evolution explains novel body shape in a Triassic coelacanth from Switzerland
Lionel Cavin, Bastien Mennecart, Christian Obrist, Loïc Costeur & Heinz Furrer
Scientific Reports 7, Article number: 13695 (2017)
Scientific Reports 7, Article number: 13695 (2017)
doi:10.1038/s41598-017-13796-0
Abstract
A
bizarre latimeriid coelacanth fish from the Middle Triassic of
Switzerland shows skeletal features deviating from the uniform anatomy
of coelacanths. The new form is closely related to a modern-looking
coelacanth found in the same locality and differences between both are
attributed to heterochronic evolution. Most of the modified osteological
structures in the new coelacanth have their developmental origin in the
skull/trunk interface region in the embryo. Change in the expression of
developmental patterning genes, specifically the Pax1/9 genes,
may explain a rapid evolution at the origin of the new coelacanth. This
species broadens the morphological disparity range within the lineage of
these ‘living fossils’ and exemplifies a case of rapid heterochronic
evolution likely trigged by minor changes in gene expression.
Introduction
Coelacanth fishes, or actinistians, are represented by the living genus Latimeria
and by about 50 extinct genera ranging from the Early Devonian to the
Late Cretaceous. The extant coelacanths are commonly qualified as
‘living fossils’ because of the monotonous morphological disparity they
display during their evolutionary history. Indeed anatomically modern
coelacanths are known since the Early Devonian1 and only a few morphological deviating genera are recorded in the Middle – Late Devonian and in the Early Carboniferous2.
Here, we describe a coelacanth from the Middle Triassic of Switzerland
which shows highly derived anatomical features in the posterior moiety
of the skull, the pectoral girdle and the lower jaw. A phylogenetic
analysis places the new form as the sister-genus of Ticinepomis, a
latimeriid found in the same formation. Differences between both genera
are attributed to heterochronic evolution. Most of the modified
anatomical structures in the new coelacanth have their developmental
origin in the skull/trunk interface region in the embryo. Several
patterning genes affect this region of the embryo during development3,4. Among them, Pax1/9
genes code for transcription factors required for the development of
teeth and skeletal elements of the skull, vertebrae, pectoral girdle and
limbs, and a change in their expression may explain a rapid evolution
at the origin of the new coelacanth.
Sarcopterygii Romer, 1955
Actinistia Cope, 1891
Latimeriidae Berg, 1940 sensu Dutel et al., 20125
Foreyia gen. nov.
Sarcopterygii Romer, 1955
Actinistia Cope, 1891
Latimeriidae Berg, 1940 sensu Dutel et al., 20125
Foreyia gen. nov.
Diagnosis
Latimeriid
coelacanth with dermal bones covered with numerous large tubercles;
hypertrophied otico-occipital portion of skull; fusion of postparietal,
supratemporal and extrascapular in postparietal shield, which forms a
dome in occipital region; supraorbital sensory canal running in a wide
groove; short and curved mandible; pterygopalatine deeper than long with
enlarged autopalatine; lachrymojugal and squamosal fused; hypertrophied
clavicle; few abdominal vertebrae (seventeen); expanded dorsal and
caudal fins; and atrophied pectoral fins.
Foreyia maxkuhni gen. et sp. nov.
Foreyia maxkuhni gen. et sp. nov.
Etymology
The
generic name honors late Peter L. Forey for his contribution on the
study of coelacanth fishes. The specific epithet refers to Max Kuhn, who
kindly supported for 12 years the preparation and study of fossils from
the Middle Triassic of Graubünden and especially the specimens
described here.
Holotype
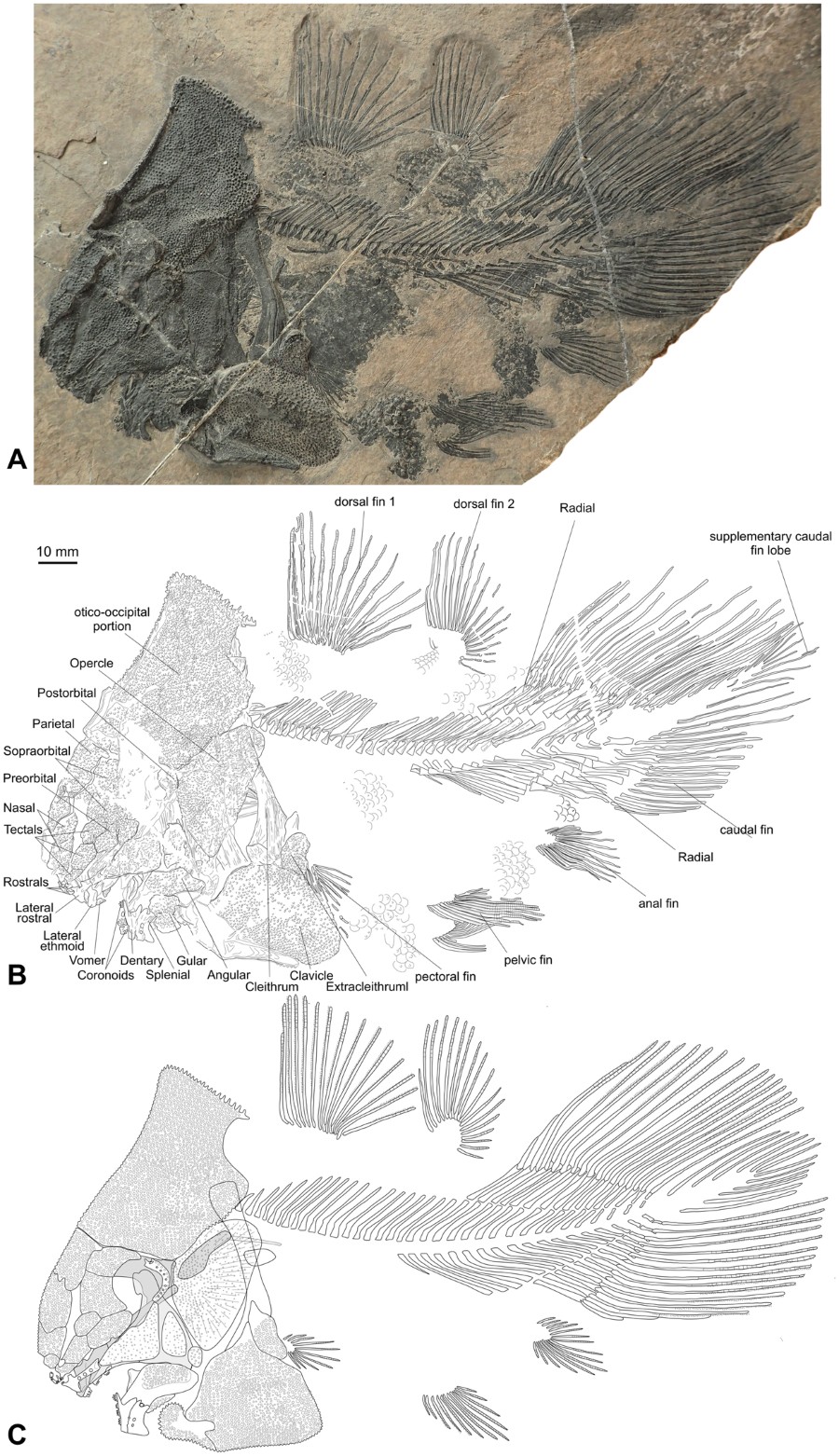
A complete specimen preserved in left lateral view (PIMUZ A/I 4620) (Figs 1, 2C, S2, S4, S6).
Skeleton of the new coelacanth Foreyia maxkuhni gen. et sp. nov. (A) Photo and (B) outline of the holotype (PIMUZ A/I 4620). (C) Reconstruction of the whole skeleton.
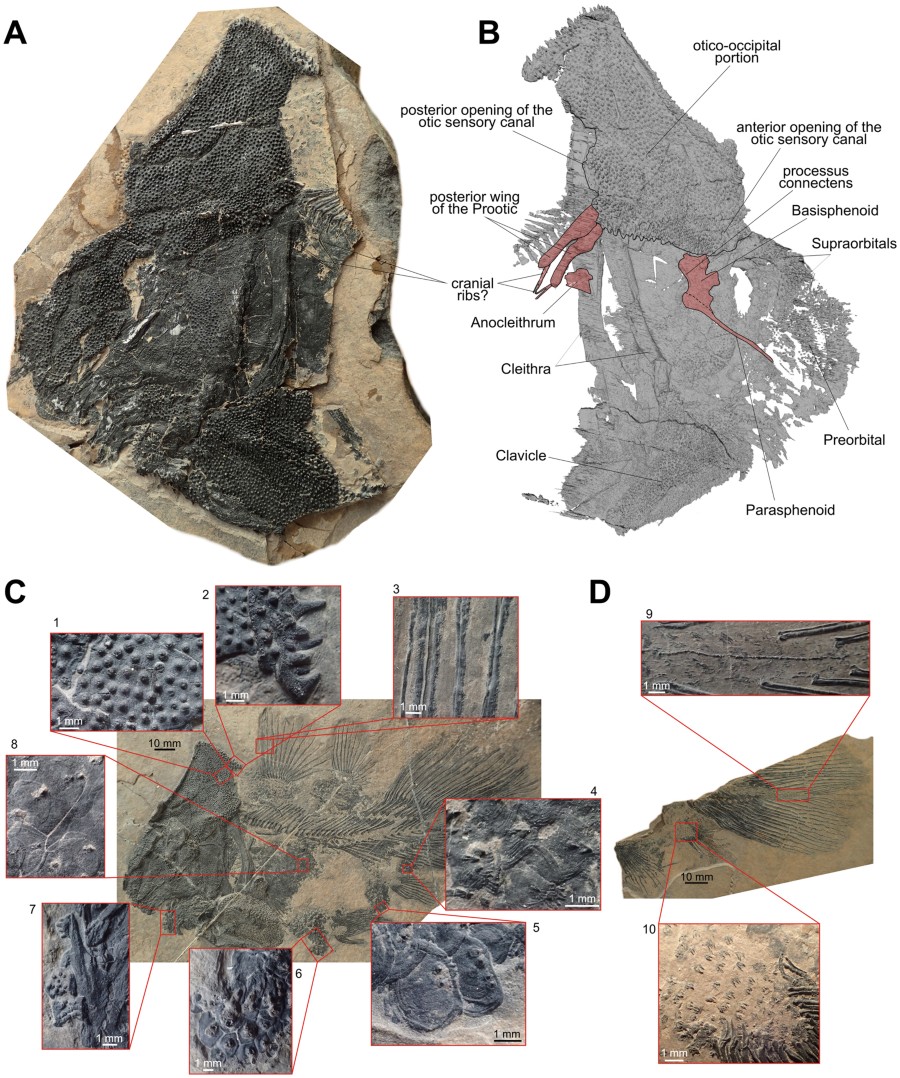
Osteological details of the new coelacanth Foreyia maxkuhni gen. et sp. nov. (A) Photo and (B) surface CT reconstruction of the skull of the paratype (PIMUZ A/I 4372). (C) Tubercles and denticles in the Holotype (PIMUZ A/I 4620) and (D)
in the paratype (PIMUZ A/I 4372). 1, tubercles on the skull roof. 2,
large spine-like tubercles on the posterior margin of the
otico-occipital shield. 3, denticles on the fin rays of the first dorsal
fin. 4, scales with denticles from the ventral margin of the caudal
peduncle. 5, scales with denticles from the anal region. 6, scales with
denticles from the belly region. 7, toothed coronoid bones. 8, scales
with denticles from the flank. 9, supplementary caudal fin lobe with
spiny scales. 10, Scales with denticles from the lobe of the anal fin.
Diagnosis
As for genus, single species.
Description
A detailed description of Foreyia is available in online content (Supplementary Information and Figs S2–S6).
Only features departing from generalized coelacanths are mentioned
herein. The skull roof of the posterior part of the otico-occipital
portion of the neurocranium is circa 1.5 times longer than the skull
roof of the ethmosphenoid portion. In most actinistians, the
ethmosphenoid portion is significantly longer, between 1.5 to 2 times,
than the otico-occipital portion. In a few Palaeozoic genera, the
otico-occipital portion is almost as long as the ethmosphenoid portion (Caridosuctor, Rhabdoderma) or is even slightly longer (Miguashaia, Diplocercides, Sassenia), but never in the proportions seen in Foreyia
(1.5 times longer). All the bones of the skull roof, the angular bone
in the lower jaw and the clavicles are covered with densely packed large
tubercles, while cheek and opercular bones are covered with smaller and
less densely packed tubercles (Fig. 2C). Foreyia
is unique among coelacanths by its proportionally huge postparietal
shield, which forms a dome in this fish and mirrors the ventral
hypertrophied clavicle. No limits between ossifications are visible
within the postparietal shield, neither with optical instruments nor
with CT images (Figs 2B, S5, Smovie).
We hypothesize that the postparietal shield is composed of a single,
paired or unpaired ossification resulting from the complete fusion of
the original ossifications (postparietals, supratemporals and
extrascapulars). The skull roof of the parietonasal shield of Foreyia
is typical for coelacanths, except the supraorbital sensory canal,
which ran in a wide groove between the medial and the lateral series of
bones and the ethmoid region, which is short. CT images of the paratype
(PIMUZ A/I 4372) shows embedded in the matrix two rounded processes
extending posteriorly from the postparietal shield and overpassing
posteriorly the cleithra (Figs 2B, S5).
They are interpreted as the posterior wings of the prootics, which have
shifted backward before fossilization. These wings are associated with
rod-like elements visible externally on the paratype that we tentatively
identify as cranial ribs. A large triangular plate-like bone in the
cheek is interpreted as a fused lachrymojugal and jugal. The lower jaw
of Foreyia has an unusual general comma-shape, but the typical actinistian apomorphic organization is recognized (Fig. S6). The dentary is hooked-shaped as in Latimeria and other derived coelacanths.
The shoulder girdle of coelacanths is said to be remarkably conservative, except in Miguashaia8 and, now, Foreyia. Contrary to all other coelacanths, which show a gap between the skull and the pectoral girdle, the cleithrum in Foreyia is situated at the level of the otico-occipital moiety. CT images show the dorsal extremities of the cleithra positioned against the postparietal shield (Figs 2B, S5), but the exact nature of the connection between the pectoral girdle and the skull cannot be observed. No anocleithra are visible externally, but the CT scan shows in the matrix a paired ossification oriented posteriorly and located on the internal side of the cleithrum in the mid-depth of the vertical branch (Fig. 2B; Fig. S5). Although the shape and the location are unusual for coelacanths, these bones are regarded as modified anocleithra. The ventral half of the cleithrum is hidden under the hypertrophied clavicle completely covered with the same strong ornamentation as present on the skull roof. A reniform extracleithrum covered by the same kind of tubercles borders a concavity of the posterodorsal corner of the clavicle. Its large ovoid shape is more reminiscent of the extracleithrum of the basal Miguashaia rather than that of the more derived genera, in which it is much slender9,10. A probable interclavicle is fused through a V-shaped suture to the anteroventral tips of both clavicles. Most coelacanths have no interclavicle, except Whitheia and Laugia, in which it is a small subdermal ossification of probable endochondral origin8, and Miguashaia, in which it bears ornamentation and has a dermal origin10. The scales bear two to four spines and those from the belly seem to form a paving-like structure, which may have acted as a kind of armoured protection. The postcranial skeleton of Foreyia fits the general Bauplan of coelacanths, except meristic features and fin size proportions. The paired fins are characterized by low number of fin rays: ten rays in the pectoral fins (only Allenypterus has less rays (9)) and 12 rays in the pelvic fins (Allenypterus has less rays (6) and Hadronector has the same number). To the contrary, the dorsal and caudal fins are proportionally overdeveloped in Foreyia. The numbers of rays in these fins are in the range of other coelacanths, except for the anterior dorsal, which has the highest number together with Allenypterus (15). The total number of vertebrae is the lowest known among coelacanths due to an unusually low number of abdominal vertebrae (17).
The shoulder girdle of coelacanths is said to be remarkably conservative, except in Miguashaia8 and, now, Foreyia. Contrary to all other coelacanths, which show a gap between the skull and the pectoral girdle, the cleithrum in Foreyia is situated at the level of the otico-occipital moiety. CT images show the dorsal extremities of the cleithra positioned against the postparietal shield (Figs 2B, S5), but the exact nature of the connection between the pectoral girdle and the skull cannot be observed. No anocleithra are visible externally, but the CT scan shows in the matrix a paired ossification oriented posteriorly and located on the internal side of the cleithrum in the mid-depth of the vertical branch (Fig. 2B; Fig. S5). Although the shape and the location are unusual for coelacanths, these bones are regarded as modified anocleithra. The ventral half of the cleithrum is hidden under the hypertrophied clavicle completely covered with the same strong ornamentation as present on the skull roof. A reniform extracleithrum covered by the same kind of tubercles borders a concavity of the posterodorsal corner of the clavicle. Its large ovoid shape is more reminiscent of the extracleithrum of the basal Miguashaia rather than that of the more derived genera, in which it is much slender9,10. A probable interclavicle is fused through a V-shaped suture to the anteroventral tips of both clavicles. Most coelacanths have no interclavicle, except Whitheia and Laugia, in which it is a small subdermal ossification of probable endochondral origin8, and Miguashaia, in which it bears ornamentation and has a dermal origin10. The scales bear two to four spines and those from the belly seem to form a paving-like structure, which may have acted as a kind of armoured protection. The postcranial skeleton of Foreyia fits the general Bauplan of coelacanths, except meristic features and fin size proportions. The paired fins are characterized by low number of fin rays: ten rays in the pectoral fins (only Allenypterus has less rays (9)) and 12 rays in the pelvic fins (Allenypterus has less rays (6) and Hadronector has the same number). To the contrary, the dorsal and caudal fins are proportionally overdeveloped in Foreyia. The numbers of rays in these fins are in the range of other coelacanths, except for the anterior dorsal, which has the highest number together with Allenypterus (15). The total number of vertebrae is the lowest known among coelacanths due to an unusually low number of abdominal vertebrae (17).
Discussion
Phylogenetic relationships
At first sight, the highly-modified coelacanth Foreyia
recalls basal Palaeozoic coelacanths. In particular, its general head
morphology and some meristic features are reminiscent of the
Carboniferous Allenypterus, such as a steep and convex profile of
the anterior moiety in lateral view and a proportionally short and deep
mandible. Its pectoral girdle shares superficial characters with the
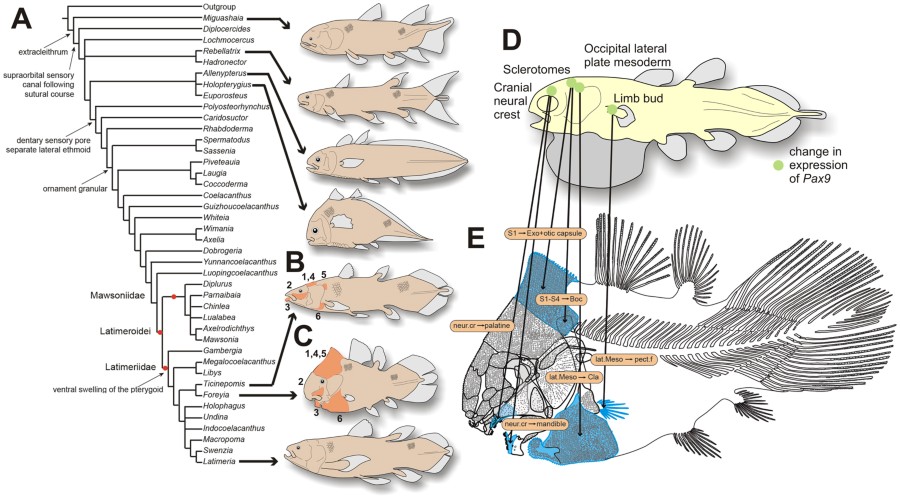
Devonian Miguashaia. However, a cladistic analysis places Foreyia as the sister-taxon of Ticinepomis11, a genus recovered from the same formation at a nearby locality12 (Figs 3A, S7). Both genera are nested within the latimeriids. The node supporting the Latimeria – Foreyia clade is weakly supported but Ticinepomis shares with Foreyia other characters not included in the cladistic analysis (Fig. 2B,C). These are: 1) The postparietal shield of Ticinepomis is proportionally smaller than in Foreyia, but no sutures are visible between the postparietal and supratemporal ossifications as in Foreyia; 2) The lachrymojugal and squamosal are poorly preserved and fragmented in the holotype of T. peyeri.
A possible reconstruction based on direct observation of the holotype
is to regard these fragments as belonging to a single large triangular
plate corresponding to the fusion of the lachrymojugal and squamosal, as
in Foreyia; 3) The lower jaw of Ticinepomis is less derived than that of Foreyia.
However, the dentary and the splenial of the former are both angled,
reminiscent of the curved mandible of the latter; 4) The ornamentation
of most of the dermal bones consists in both genera of tubercles,
although in Ticinepomis they are smaller; 5) A broad dorsal
extremity of the cleithrum is present in both genera; 6) A massive
ornamented clavicle is present in both genera, but in a much more
important proportion in Foreyia than in Ticinepomis.
Phylogenetic relationships of Foreyia maxkuhni gen. et sp. nov. and developmental origin of the derived characters. (A)
Strict consensus trees of the 259 most parsimonious trees of 317 steps
(CI = 0.3817, RI = 0.6766) with some of the uniquely derived characters
present in Foreyia maxkuhni on the left, and reconstructions of genera with atypical general morphology. (B and C) Shared features of Ticinepomis peyeri and Foreyia maxkuhni (in orange) not included in the cladistics analysis (see main text for numbers). (D)
Reconstruction of a coelacanth embryo with localization of embryonic
tissues that give rise the derived skeletal features present in Foreyia. It is hypothesized that changes in the expression of Pax9 may have altered the derived characters shown in blue on the reconstruction (E).
All the drawings were made by LC. Abbreviation: Boc, basioccipital;
Cla, clavicle; Exo, exoccipital; lat. Meso., lateral mesoderm; neur.
cr., neural crest; pect. f., pectoral fin; S (numbered), somite.
Heterochronic evolution and its developmental basis
Most of the shared features in Ticinepomis and Foreyia
are more weakly developed in the former than in the latter genus, and
they indicate a possible heterochronic evolution at the origin of Foreyia. This hypothesis is strengthened by the fact that the general coelacanth skeletal organization is not altered in Foreyia, but only relative bone sizes vary compared to the generalized coelacanths Bauplan
(hypertrophied occipital and clavicular regions, comma-shaped mandible,
few abdominal vertebrae and rays in paired fins, and dense covering of
large tubercles on the dermal bones and denticles on the scales).
Several of these features are developmentally linked in sarcopterygians
and, compared with extant models, partly under the control of the same
genes. In the chick embryo, the anterior most somites give rise to part
of the otic capsule and the exoccipital bone (somite 1) and to the
basioccipital bone (somites 2–4)13.
The occipital lateral plate mesoderm at the level of somites 1–3 gives
rise to the ventromedial extremity of the clavicle in amniotes, which is
regarded in part as homologous to the dermal clavicle of bony fishes3,4.
Although numerous developmental patterning genes have a control on
these features, the best candidate is the paired box gene 9, or Pax9, widely distributed among vertebrates and present in Latimeria14,15 (alternative genes, such as Prrx1/Prrx2, HoxD, Tbx14
are discussed in Supplementary Information). In extant bracketing
clades of coelacanths, chondrichthyans and amniotes, the embryonic
expression of Pax9 occurs at the level of the head mesoderm, of
the sclerotomes (those from the first somites give rise to the occipital
bones), of the postotic mesoderm (gives rise to the clavicle) and of
the trunk mesoderm (gives rise to paired limbs), as well as at the level
of the neural crest (give rises to odontodes)16,17. Pax9
expression on the neural crest at the level of the first rhombomeres
also affects the palatine and the coronoid regions in the mouse16, two anatomical domains also modified in Foreyia. Although Pax9 in deficient mice does no show phenotypic features directly linkable to the peculiar morphology of Foreyia, the targeted embryological tissues make this gene potentially at the origin of its heterochronic evolution (Fig. 3D,E). Pax9 regulates synergetically the development of the vertebral column with Pax1. The latter has a more limited expression than Pax9
in amniotes and has an effect on the development of the pectoral
girdle, particularly on the acromion, which is a process on the scapula
connecting the clavicle18,19. The acromion is mesodermal in origin3, as is the hypertrophied clavicle of Foreyia. It is possible that in coelacanths the expression Pax1 and Pax9 are more similar between them than they are in amniotes, as it is the case in the ray-fin fish Medaka20.
In this case, both genes should be considered together in their effects
on the phenotype. The search of a single genetic source is an
oversimplification since we know that Pax genes work in cooperation with Hox genes21,22. The developmental and genetic pathways proposed here suggest that the bizarre morphology of Foreyia (Fig. 4) might be the consequence of a rapid heterochronic evolution.
Reconstruction of the living coelacanth Foreyia maxkuhni gen. et sp. nov. Artwork by Alain Bénéteau.
Methods
Fossil preparation
Both new specimens of coelacanth were found during systematic bed-by-bed excavations in the upper part of the Prosanto Formation by Christian Obrist, under direction of Heinz Furrer. Both specimens were found broken in several fragments, then glued together and very carefully prepared mechanically with air-tool, fine sharp steel needles and sand-blaster by C.O.: The holotype (PIMUZ A/I 4620), a complete skeleton found in summer 2014 (bed 141) and prepared in 2016 during 150 hours; the paratype (PIMUZ A/I 4372), a broken specimen recovered in two fragments in summer 2015 (bed 150) and prepared in 2015 during 30 hours.Computed tomography
The paratype (PIMUZ A/I 4372) of Foreyia maxkuhni was scanned with high resolution x-ray computed tomography at the Biomaterial Science Center of the University of Basel using a phoenix nanotom® (General Electric Wunstorf, Germany) equipped with a 180 kV/15 W nanofocus x-ray source. A voltage of 180 kV and current of 30 mA were used with a 0.25-mm Cu filter. 1440 poses were taken with an average of 6 images for each pose.Phylogenetic analysis
We ran the analysis using PAUP 4.0b1023 heuristic search option, random addition sequence, replicated 100 times, 10 trees held at each iteration, and tree bisection and reconnection branch swapping.Data Availability
The protocols used in the development of this study are available in the ‘Supplementary Information’ section.Additional Information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
1. Zhu, M. et al. Earliest known coelacanth skull extends the range of anatomically modern coelacanths to the Early Devonian. Nat. Commun.
3, 772 (2012).
Article
PubMed
Article
PubMed
2. Friedman,
M. & Coates, M. I. A new recognized fossil coelacanth highlights
the early morphological diversification of the clade. P. R. Soc. B
273, 245–250 (2006).
Article
CAS
Article
PubMed
PubMed Central
3. Matsuoka, T. et al. Neural crest origins of the neck and shoulder. Nature
436, 347–355 (2005).
ADSCAS
Article
PubMed
PubMed Central
4. Nagashima, H. et al. Developmental origin of the clavicle, and its implications for the evolution of the neck and the paired appendages in vertebrates. J. Anat. 229, 536–548 (2016).
Article
PubMed
5. Dutel, H. et al. The giant Cretaceous coelacanth (Actinistia, Sarcopterygii) Megalocoelacanthus dobiei Schwimmer, Stewart & Williams, 1994, and its bearing on Latimerioidei interrelationships. PLoS ONE 7, e49911 (2012).
ADS
CAS
Article
PubMed
PubMed Central
6. Bürgin, T., Eichenberger, U., Furrer, H. & Tschanz, K. Die Prosanto-Formation—eine fischreiche Fossil-Lagerstätte in der Mitteltrias der Silvretta-Decke (Kanton Graubünden, Schweiz. Eclogae geol. Helv. 84, 921–990 (1991).
7. Furrer,
H., Schaltegger, U., Ovtcharova, M. & Meister, P. U-Pb zircon age
of volcaniclastic layers in Middle Triassic platform carbonates of the
Austroalpine Silvretta nappe (Switzerland). Swiss J. Geosci.
101, 595–603 (2008).
CAS
Article
CAS
Article
8. Forey, P. L. History of the Coelacanth Fishes. 419 (Chapman and Hall, London, 1998).
9. Cloutier, R. The primitive actinistian Miguashaia bureaui Schultze (Sarcopterygii). (Verlag Dr. Friedrich Pfeil, München, 1996).
10. Forey, P. L., Ahlberg, P. E., Luksevics, E. & Zupins, I. A new coelacanth from the Middle Devonian of Latvia. J. Vertebr. Paleontol.
20, 243–252 (2000).
11. Rieppel, O. A new coelacanth from the Middle Triassic of Monte San Giorgio, Switzerland. Eclogae geol. Helv.
73, 921–939 (1980).
12. Cavin,
L., Furrer, H. & Obrist, C. New coelacanth material from the Middle
Triassic of eastern Switzerland, and comments on the taxic diversity of
actinistans. Swiss J. Geosci.
106, 161–177 (2013).
Article
Article
13. Couly, G. F., Coltey, P. M. & Le Douarin, N. M. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117, 409–429 (1993).
CAS
PubMed
14. Amemiya, C. T. et al. The African coelacanth genome provides insights into tetrapod evolution. Nature 496, 311–316 (2013).
ADS
CAS
Article
PubMed
PubMed Central
15. Paixão-Côrtes, V. R., Salzano, F. M. & Bortolini, M. C. Evolutionary history of chordate PAX genes: dynamics of change in a complex gene family. PloS ONE 8, e73560 (2013).
ADS
Article
PubMed
PubMed Central
16. Peters, H., Neubüser, A., Kratochwil, K. & Balling, R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Gene. Dev.
12, 2735–2747 (1998).CAS
Article
PubMed
PubMed Central
Article
PubMed
PubMed Central
17. Adachi, N., Takechi, M., Hirai, T. & Kuratani, S. Development of the head and trunk mesoderm in the dogfish, Scyliorhinus torazame: II. Comparison of gene expression between the head mesoderm and somites with reference to the origin of the vertebrate head. Gene. Dev.
14, 257–276 (2012)
18. Timmons, P. M., Wallin, J., Rigby, P. & Balling, R. Expression and function of Pax 1 during development of the pectoral girdle. Development 120, 2773–2785 (1994).
CAS
PubMed
19. Wilm, B., Dahl, E., Peters, H., Balling, R. & Imai, K. Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. P. Natl. Acad. Sci. USA 95, 8692–8697 (1998).
ADS
CAS
Article
20. Mise, T., Iijima, M., Inohaya, K., Kudo, A. & Wada, H. Function of Pax1 and Pax9 in the sclerotome of medaka fish. Genesis
46, 185–192 (2008).
CASArticle
PubMed
21. Aubin, J., Lemieux, M., Moreau, J., Lapointe, J. & Jeannotte, L. Cooperation of Hoxa5 and Pax1 genes during formation of the pectoral girdle. Dev. Biol.
244, 96–113 (2002).
CASArticle
PubMed
22. Casaca, A., Santos, A. C. & Mallo, M. Controlling Hox gene expression and activity to build the vertebrate axial skeleton. Dev. Dynam.
243, 24–36 (2014).
CASArticle
23. Swofford, D. L. PAUP*: Phylogenetic Analysis Using Parsimony and Other Methods (Software). (Sinauer Associates, Sunderland, 2001).
Acknowledgements
The
Palaeontological Institute and Museum, University of Zürich (PIMUZ)
enabled H.F. to conduct systematic prospecting and numerous excavations
near Davos. The government of Canton Graubünden, the municipality of
Davos, and the Bündner Naturmuseum in Chur gave permission for the
excavations and financial support. Max Kuhn (Uster) provided generous
financial support for the preparation of the specimens by C.O. B.M. and
L.Ca. also thank the Département de la culture et du sport de la Ville
de Genève for a financial support for computer facilities, and Philippe
Wagneur (Natural History Museum of Geneva) for assistance to produce the
CT scan movie. We thank Anne Kemp (Griffith University) and Mélanie
Debiais-Thibaud (University of Montpellier) for discussion. This paper
is a contribution to the project “Evolutionary pace in the coelacanth
clade: New evidence from the Triassic of Switzerland” supported by the
Swiss National Science Foundation (200021-172700) by L.Ca.
Author information
Affiliations
Department of Geology and Palaeontology, Muséum d’Histoire Naturelle, CP6434, 1211, Geneva, 6, Switzerland
- Lionel Cavin
Naturhistorisches Museum Basel, Augustinergasse 2, 4001, Basel, Switzerland
- Bastien Mennecart
- & Loïc Costeur
Erliackerweg 8, 4462, Rickenbach, BL, Switzerland
- Christian Obrist
Paläontologisches Institut und Museum der Universität Zürich, Karl Schmid-Strasse 4, 8006, Zurich, Switzerland
- Heinz Furrer
Contributions
L.Ca. wrote the description of the new taxon, collected and analyzed the phylogenetic and ontogenetic data, and wrote the corresponding parts of the manuscript. C.O. collected specimens PIMUZ A/I 4620 and PIMUZ A/I 4372, and prepared them. H.F. analyzed the stratigraphic data in the field, and wrote the corresponding methods and results. B.M. and L.Co. performed the CT scan analysis and interpreted the images. L.Ca. and H.F. obtained funding for fieldwork and data analysis. All authors contributed to write the last version of the text.Competing Interests
The authors declare that they have no competing interests.Corresponding author
Correspondence to Lionel Cavin.Electronic supplementary material
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.