Christadelphian special creationist John Collyer's fourteenth '20 scientific fact seldom taught to students' asserts that "amino acids formed synthetically are either right handed or left handed." He continued by declaring that the "amino acids in all forms of life are left-handed, without exception, are evidence of intelligent selection and design." Once more, Collyer like most special creationists has conflated evolution and abiogenesis. Furthermore, he is invoking the argument from personal incredulity. Finally, his argument betrays ignorance of abiogenesis research.
The 'handedness' of amino acids is referred to as their chirality. Amino acids have two forms: L-amino and D-amino:
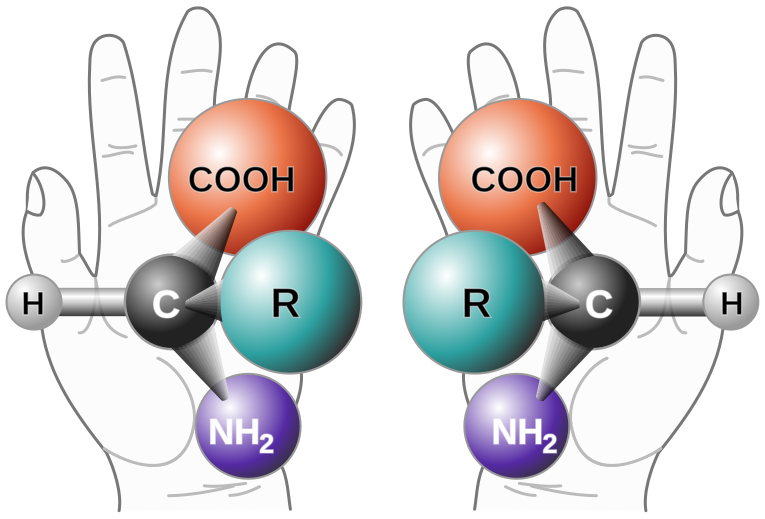 |
| Source: Wikipedia |
Living creatures almost always synthesise L-amino acids and D-sugars. L and D are short for levo (left handed) and dextro (right handed) respectively. How this occurred is still an open question, and is known in abiogenesis research as the homochirality problem. [1]
Two commonly advanced explanations for the homochirality problem are:
- Extraterrestrial origin: The Murchison meteorite has an imbalance in the handedness of the amino acids found on it. Circularly polarised light in space can trigger the formation of optical isomers, which provides a ready explanation for the origin of amino acid homochirality. [2]
- Chiral amplification: in some autocatalytic systems, a slight imbalance in the handedness of amino acids can lead to a marked imbalance in the handedness of the amino acids at the end of the reaction. [3]
One argument against the first option is that while circularly polarised light does trigger optical isomer formation, the amount of amino acids delivered to Earth via meteorites would have been trivial. The biochemist Larry Moran (who is an atheist and strong critic of special creationism, as well as a sceptic of the 'prebiotic soup' hypothesis) points out that:
The current flux of extraterrestrial organic material is about 3 × 10^8 grams per year from cosmic dust and micrometeorites. About 1% of this is amino acids and most of them are not the ones found in living organisms. This should give rise over time to a concentration in the oceans of about 0.1 nM (10^-10 M). That's not sufficient for life to have originated.
The flux in the past was almost certainly much greater and lots of organic material might have been delivered by large meteorites; however, it's unlikely that amino concentrations in the oceans could ever have been more than 10-100 pM for all amino acids combined. [4]
Moran argues that the 'metabolism-first' hypothesis (which he favours) provides a ready explanation for the origin of homochirality:
One of the advantages of the metabolism first scenario is that it offers a simple "solution" to the chirality/racemization problem by explaining why all naturally occurring amino acids are left-handed [see Can watery asteroids explain why life is 'left-handed'?]. Another advantage is that it doesn't require spontaneous formation of nucleotides—a major limitation of the RNA world scenario since spontaneous formation of such molecules is very improbable. [5]
Microbiologist Rosie Redfield in fact argues that the origin of homochirality poses no major problems for origin of life research:
The fundamental argument seems to be that some special forces or factors are needed to explain how and/or why the first living things used only D-sugars and only L-amino acids. It's often claimed that this would not have happened unless the starting abiotic materials had an excess of one enantiomer (definition below) over the other.
I think people have fallen into the error of assuming that, at the molecular level, enantiomers are much more similar to each other than to other related molecules. But it shouldn't be any harder for an asymmetric reactant or catalyst to distinguish D-glucose from L-glucose than from either enantiomer of fructose or galactose. Or to distinguish L-leucine from D-leucine than from D- or L-isoleucine. They may contain the same atoms but they all have entirely different shapes, and so they are all entirely different molecules...
Chemists discovered chirality when investigating the bulk properties of pure crystals and solutions. Chemical synthesis of a chiral molecule from simpler non-chiral precursors usually produces equal amounts of both enantiomers, whose identical physical properties make them very difficult to separate. Chemists thus view enantiomers as almost-identical molecules, differing only in their 'handedness'.
But chemists are late arrivals on the evolutionary scene, and the first self-replicating entities would usually have interacted with individual molecules in complex mixtures. Because all but the simplest of biologically relevant molecules are asymmetric, most inter-molecular interactions would always have been between asymmetric participants, each no more likely to confuse their partner with its enantiomer than with any other molecule. The fact that crystals of D-glucose and L-glucose have the same bulk properties (solubility, melting temperature) would have been irrelevant.
We don't need to fuss with defining 'life', but can simply think about the origin of entities capable of evolving by natural selection (having heritable variation causing differential reproduction). Any molecule complex enough to have heritable variation would certainly have been complex enough to be asymmetric. To such molecules, discrimination between enantiomers wouldn't have been any more of a problem than discriminating between other possible reactants. [5]
This is not to argue that the question of the origin of homochirality has been answered. It has not. However, there are a number of well-regarded, physically plausible mechanisms to explain it, and for Collyer to claim that the origin of homochirality in amino acids is a mystery is simply wrong, and demonstrates no knowledge of the primary literature.
References
1. Collyer's dogmatic assertion that amino acids are all exclusively left-handed is not true in that while all the amino acids in proteins made in the cell are left-handed, D-amino acids, although rare, do occur in nature, and can be formed as a result of enzymal post-translation modification. This does not affect the homochirality problem, but once more shows the depths of Collyer's ignorance on the subject.
2. This is a large meteorite, weighing over 100kg which was found in Murchison, Victoria in 1969. It is rich in organic compounds, including over 100 amino acids. Some of these are present in enantiomeric excess, that is, with a bias towards amino acid handedness.
3. Meierhenrich U, Amino Acids and the Asymmetry of Life (2008, Springer Verlag)
4. Moran L "Can watery asteroids explain why life is 'left-handed'?" Sandwalk April 24 2009
5. Redfield R "Solving an unnecessary problem: chirality and the origin of life" RRResearch Jan 18 2011